Production
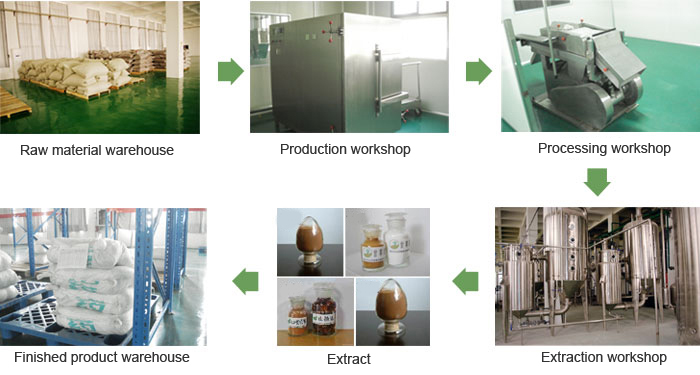
Anhui GYT Chinese Medicine Co. Limited has Chinese medicine production line with Chinese herbal medicine workshop, workshop for Chinese medicine extraction, oral solid preparation workshop, functional beverage plant and supporting material library, pieces of finished product storehouse, sewage treatment plants and other factories and central air conditioning, air pressing water, such as sewage treatment system and plant design, equipment, facilities, purification installation that are in line with the new GMP standards.
Company quality control of production process
1.Establishment of Chinese herbal medicine planting bases
In order to ensure the quality of Chinese medicinal materials, the company began to control the quality of medicinal materials from the source. The company established Chinese herbal medicine base, set up a base management organization, cleared the responsibilities of departments, and trainied the related personnel for GAP knowledge.
In a file management, according to the good agricultural practice standard released by the State Administration of quality supervision and the National Standardization Management Committee, from "the second part about farm base control points and compliance criteria, the third part about crop base control points and compliance criteria, the fifth portions of fruit and vegetable control points and compliance criteria", our company drew up the directive rules for good agriculturre, which includes the document control procedures, recording and storage management procedures, product quality and security traceability control procedures, environmental management procedures, product recall procedures, complaints and complaints handling procedures, staff health, safety and welfare programs, internal audit procedure, good agricultural standardized internal penalty etc. And our company made a series of management procedures: employee health management, base health management, fertilizer using rules, visitors safety regulations, pesticide management approach, fertilizer management approach, spraying equipment management system, abandoned agricultural equipment management system, base staff health and safety policy, the relevant provisions on the welfare of workers, base accident disposal, environmental protection regulations, wildlife protection, protective clothing and cleaning procedure, and safety protection operation manual to strictly ensure their implement.
In the field of management on the basis of standard terms and GAP implementation manual, company has the scientific management, that is, procuring seed, fertilizer, plant protection products from the formal business, and then makes the soil preparation, seeding, transplanting and field management, and the company sent technical personnel to the base to guide medicinal material recovery to ensure no loss of the active ingredients of medicinal herbs. In production processing, the company does not use banned pesticide, in strict accordance with the medicinal grade size grading, sunning to earth, timely drying with drying methods in general to take the natural drying method, in the packaging we use clean bag packing, and then ship back to the company.
2.Direct acquisition from local areas
Outside of the base varieties, company sends business personnel to origin acquisition where they make the medicinal traits and the authenticity of identification, and send those to meet the requirements, and the representative samples to the test center for inspection of the inner quality. According to the "Chinese Pharmacopoeia"and customer needs, we test on the active ingredients of medicinal herbs, heavy metals, pesticide residues, and then purchase those qualified. We make the detection of qualified again after they are transported to the company, and make preliminary processing of those qualified. And store them according to the medicine storage method, and process them according to the production engineering to ensure the quality of Chinese medicinal materials to meet the requirements.
Quality certification



Earned the Title of Demonstration Enterprise

